Fingerprints have been a staple of forensic science for decades, providing a reliable (though not perfect) means of identifying suspects and placing people at crime scenes. In recent years, scientists have turned their attention towards exploring the additional information that could be extracted from fingerprints, in particular, chemical information for police intelligence. Fingerprints are composed of a mixture of chemical compounds both excreted through our skin and picked up from the environment.
Over the past decade, analytical chemists have shown that there may be sufficient chemical differences in the fingerprints of male and female donors to differentiate between the sexes. By studying these chemical differences, it may be possible to build a model capable of predicting the sex of a fingerprint donor based on the chemical compounds within the fingerprint.
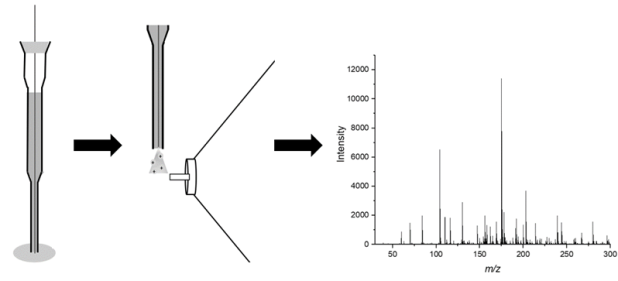
In a recent study published in Forensic Chemistry, a method was developed to predict the sex of a donor based on the presence of peptides and proteins in fingermarks. The study used MALDI-MS (Matrix-assisted laser desorption/ionization mass spectrometry), a type of mass spectrometry that enables both the measurement and identification of the chemical constituents in a material, in addition to the imaging of samples. MALDI has already been demonstrated to be a powerful technique in the chemical analysis of fingermarks, including imaging fingermarks and detecting the presence of blood and drugs in the mark. Much of the research in the area has been led by Professor Simona Francese of Sheffield Hallam University in the UK, senior author on this recent paper.
In the study, 199 participants donated multiple fingermarks, culminating in hundreds of samples for analysis. The fingermarks used for the analysis were natural, that is to say, deposited with no fingertip preparation beforehand. Many studies in the chemical analysis of fingerprints use “groomed” marks, in which the fingers are rubbed on the face or forehead in order to load the fingers with skin secretions prior to deposition. Although this provides rich samples for analysis, they are not applicable to fingermarks encountered in the real world.
After fingermark collection, MALDI-MS was then used to analyse the samples, focusing on the measurement of peptides and proteins in the deposited marks. To further mimic realistic scenarios, fingermarks were analysed both undeveloped and enhanced by common fingerprint visualisation techniques (white powder and vacuum metal deposition). Based on the chemical profiles produced, a predictive model was then constructed for the purpose of predicting the sex of the donor of unknown fingermarks, such as those that may be discovered at crime scenes.
The technique had a predictive power of up to 86%, demonstrating the potential to differentiate between male and female donors to a degree. There were, however, challenges in this study. Polyethylene glycol (PEG)-based contaminants, routinely used in cosmetics and personal care products, were commonly encountered, interfering with the detection of the actual targets of the analysis. Furthermore, the application of fingermark development techniques also caused interference, with many mass spectra being dominated by the gold nanoparticles used in the vacuum metal deposition method. This suggests the technique may only be truly applicable in the case of undeveloped fingermarks.
Although the technique has a high predictive power, it was not able to determine the sex of the donor in all cases, rendering it unsuitable for conclusively excluding suspects from an investigation based on their sex. However the method could be used to triage fingermarks, allowing investigators to establish which marks are of the greatest importance and which should be prioritised for further study, potentially speeding up forensic investigations.
Heaton & Bury et al. Investigating sex determination through MALDI MS analysis of peptides and proteins in natural fingermarks through comprehensive statistical modelling. 2020, Forensic Chemistry, DOI: 10.1016/j.forc.2020.100271